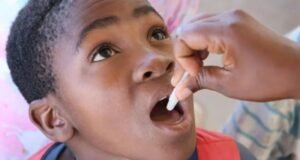
The World Health Organization (WHO) has added the LC16m8 mpox vaccine to its Emergency Use Listing (EUL), marking it as the second mpox vaccine to receive this designation following the declaration of mpox as a public health emergency of international concern (PHEIC) on August 14, 2024.
According to a statement, which came on Tuesday (Geneva local time), this decision aims to expedite vaccine access in regions experiencing surging mpox outbreaks. As of October 31, 2024, mpox cases have been reported in 80 countries, including 19 in Africa. The Democratic Republic of the Congo (DRC), the hardest-hit nation, has reported over 39,000 suspected cases and more than 1,000 deaths.
The announcement comes as Japan has pledged to donate 3.05 million doses of LC16m8, alongside specialized needles, to the DRC — the largest donation to date in response to the ongoing mpox emergency.
Developed by KM Biologics in Japan, the LC16m8 vaccine was reviewed by WHO’s Technical Advisory Group (TAG), which recommended its use for individuals aged one year and older. The vaccine is administered as a single dose via a bifurcated needle using a multiple-puncture technique.
Dr. Yukiko Nakatani, WHO Assistant Director-General for Access to Medicines and Health Products, stated, “The emergency use listing of the LC16m8 vaccine is a significant milestone in addressing the current mpox emergency, offering a new option for protection, including for children. Vaccines are crucial in the broader strategy to contain the outbreak, which also involves improved testing, diagnosis, treatment, and community engagement.”
The vaccine’s EUL assessment was based on data from the manufacturer and Japan’s Pharmaceuticals and Medical Devices Agency (PMDA). LC16m8 has been used in Japan during previous outbreaks and shown to be safe and effective, even in individuals with well-controlled HIV.
WHO’s Strategic Advisory Group of Experts (SAGE) on Immunization has recommended the vaccine for use in outbreak settings, particularly among children and those at high risk of exposure to mpox. However, the vaccine should not be used during pregnancy or by people with weakened immune systems, including those with active cancer, transplant recipients, and individuals on immunosuppressive treatments.
On September 20, 2024, the Global Advisory Committee on Vaccine Safety reviewed the latest safety data and recommended training for healthcare workers on the use of bifurcated needles to minimize risks.
WHO continues to collaborate with global partners, manufacturers, and governments to ensure the availability and safe administration of life-saving vaccines. Additionally, the WHO prequalified the Modified Vaccinia Ankara-Bavarian Nordic (MVA-BN) vaccine on September 13, 2024, expanding its use to individuals aged 12 and older by October 8, 2024.
 Weekly Bangla Mirror | Bangla Mirror, Bangladeshi news in UK, bangla mirror news
Weekly Bangla Mirror | Bangla Mirror, Bangladeshi news in UK, bangla mirror news