A new weight-loss jab will be rolled out on the NHS in England – but it could take 12 years for everyone to receive it, the NHS drugs advisory body says.
The National Institute for Health and Care Excellence (NICE) final draft guidance on Mounjaro has recommended it starts being given from March, alongside advice on diet and exercise.
It will be offered to people with a body mass index (BMI) of more than 35 and at least one obesity-related health problem – potentially 3.4 million people.
But because of concerns it could overwhelm services, and in particular GPs, NICE has agreed to give the NHS more than a decade to introduce it – an unprecedented move for a medication.
NICE chief medical officer Prof Jonathan Benger acknowledged it would mean “many people would have to wait”.
But he said: “We’ve had to make this difficult decision in order to protect vital NHS services and also to test ways of delivering this new generation of weight-loss medications.”
Patient groups have expressed disappointment at the decision to give the NHS so long.
Questions remain
Only those patients under the care of specialist weight-management services will be offered it initially – matching the approach taken with a similar weight-loss drug, Wegovy.
But from June, the NHS will start offering it to others.
It is unclear exactly how this will be done – GP practices are likely to be in charge of referring patients, but questions remain over who will be providing the ongoing support involving diet and exercise and monitoring.
NHS England is expected to publish guidance on this in the new year. It could involve the use of apps or separate services being established to support GPs.
Mounjaro, or tirzepatide, which is made by Eli Lilly, makes you feel fuller so you eat less. In trials, people on it have lost a fifth of their body weight.
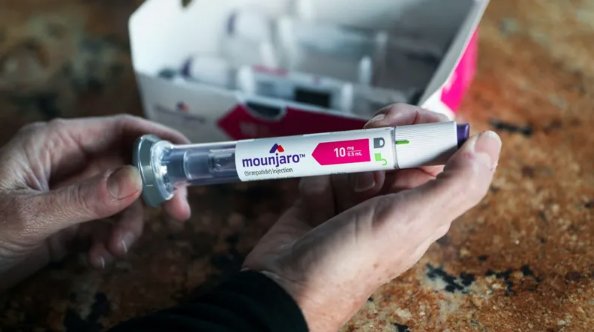
In the US, the FDA approved Mounjaro for blood sugar control in adults with type 2 diabetes in 2022
Wegovy, also known as semaglutide, which is already available on the NHS, works in a similar way. But that can only be given to those under the care of specialist weight-loss management.
There are around 40,000 people estimated to be in that position.
The drugs can be bought privately. Both medicines are also already available for people with type 2 diabetes – although semaglutide is marketed as Ozempic for those patients.
The rollout of Mounjaro opens up the option of this new generation of weight-loss drugs to the wider public who are struggling with severe obesity.
To be eligible, patients will also need to have one obesity-related condition, such as type 2 diabetes, high blood pressure or heart disease.
Under the rollout plans, those with the highest clinical need will be prioritised first.
It is estimated around 220,000 will benefit in the first three years.
NICE then plans to evaluate how that is working, before pushing ahead with the wider rollout.
As this is final draft guidance, it is still possible rollout could be delayed if there are any appeals made against the decision. If there is not, the guidance will be rubber-stamped before Christmas, NICE said.
Concerns
Mounjaro, which is given via weekly injections, will cost the NHS £122 per patient per month for the maximum dose, but NICE judged it to be cost-effective given the cost of obesity.
There is a risk that users can put weight back on once they stop using it.
Ministers in Wales will be using the guidance from NICE to inform their rollout.
It is already recommended for use in Scotland – although the NHS is reportedly struggling with its rollout.
Helen Kirrane, of Diabetes UK, said Mounjaro had a “significant” role to play in tackling obesity.
But she added: “We have concerns over the length of time it might take for people to gain access.”
Dr Kath McCullough, NHS England’s national specialty adviser for obesity, said weight-loss drugs were an
“important tool” to help tackle “one of the greatest public health issues facing the NHS”.
She said the phased rollout was needed to protect access to other NHS services that patients rely on.
And Prof Kamila Hawthorne, of the Royal College of GPs, warned the drug should not be seen as a “silver bullet”, adding that it did not come without risk and would not be the right course of treatment for everyone who is eligible.
 Weekly Bangla Mirror | Bangla Mirror, Bangladeshi news in UK, bangla mirror news
Weekly Bangla Mirror | Bangla Mirror, Bangladeshi news in UK, bangla mirror news